Instruction For Use
Fiorage Products
Ingredients
Cross-linked hyaluronic acid in Fiorage S | 16 mg |
Cross-linked hyaluronic acid in Fiorage M | 20 mg |
Cross-linked hyaluronic acid in Fiorage L | 24 mg |
Cross-linked hyaluronic acid in Fiorage XL | 26 mg |
Lidocaine hydrochloride | 3 mg |
Phosphate buffer and Mannitol (pH 7.2) | q.s .1 g |
The Fiorage syringes are sterilized by moist heat.
The needles are sterilized by radiation.
Indications
Fiorage S is an injectable hyaluronic acid gel to fill wrinkles by injection into the dermis. It is also used to outline and correct the shape of the lips.
Fiorage M is an injectable hyaluronic acid gel for lip volume augmentation, natural correction of superficial lines and wrinkles even the deepest of wrinkles away from the mouth and restoration of volume in the back of the hand by subcutaneous injection.
Fiorage L is an injectable hyaluronic acid gel to fill wrinkles and deep pits by injection into the dermis and also restoration of volume in the back of the hand by subcutaneous injection.
Fiorage XL is an injectable hyaluronic acid gel that can be injected into the deep dermis or subcutaneously for the restoration or augmentation of facial volume.
The presence of lidocaine is meant to reduce the patient’s pain during treatment.
Mechanism of action
After injection, Fiorage S/M/L/XL gel improves the volume of the treated area; then the gel is slowly absorbed over time. The duration of efficacy depends on the skin type and the injection depth. Therefore, depending on the characteristics of the treated area and the depth of injection, one to two sessions are required to achieve the optimal result. The desired correction can be made more durable through regular sessions.
Contraindications
Fiorage should not be used in the following cases:
- Areas with inflamed and/or inflected skin (such as hypertrophic scars, acne, herpes, etc.).
- Simultaneously with laser therapy or deep chemical scaling, in case of a significant inflammatory reaction, it is recommended to avoid injection of the product.
- Patients with a history of allergy to one of the ingredients.
- Patients with a history of sensitivity to hyaluronic acid and/or proteins in Gram-positive bacteria (streptococci).
- Patients with a history of allergy to lidocaine or local anesthetic of amide compounds.
- Patients with porphyria.
- Pregnant or lactating women.
- Children.
- Avoid using Fiorage L/XL for treatment of superficial wrinkles with thin skin (such as the forehead and the area around the eyes, including the eyelids, dark circles under the eyes, crow’s feet around the outer corners of the eyes), areas exposed to the vascular system (such as the glabella) and also lips.
- Avoid injection of Fiorage M into the upper eyelids.
Side Effects
The patient should be informed about potential undesirable effects resulting from the use of the product, which may occur immediately or after a certain period. Some adverse reactions are:
· Inflammatory reactions (redness, edema, urticaria, etc.) accompanied by pruritus or pressure on pain or both. These reactions may last up to one week;
· Induration or nodules in the injection area;
· Stains and color changes around the injection area, specifically after the injection of the hyaluronic acid fillers into the epidermis or thin skin (the Tyndall effect);
· Some rare, but serious, side effects after intravenous injection of dermal fillers into the face and compressed tissues: temporary or permanent visual impairment, blindness, cerebral ischemia (insufficient blood supply to the brain), and cerebral hemorrhage, with a chance of stroke, skin necrosis, and damage to underlying structures.
· Hematoma.
· Local mobility of the implant.
· Other adverse side effects associated with Fiorage injection should be reported to the distributor or manufacturer.
Method of Administration
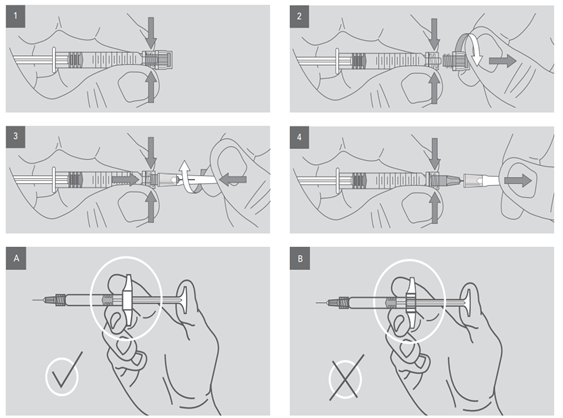
The correct way to assemble the needle head:
Figure 1) Hold the Luer-Lock of the syringe firmly with the help of the index finger and the thumb.
Figure 2) grasp the protective cap with the other hand and unscrew it.
Figure 3) while holding the Luer-Lock with your thumb and forefinger, carefully twist the tip of the needle clockwise with the other hand on the Luer-Lock until you feel a reverse pressure (This pressure can be felt at the edge of the second thread).
· Make sure that the end edge of the needle matches the Luer-Lock threads correctly, and then continue screwing the needle. Over-tightening of the needle may lead to the Luer-lock moving and leakage from the syringe during injection.
Figure 4) keep the syringe in one hand, remove the needle guard with the other hand according to the figure, and pull your hand in opposite direction to expose needle.
Figure A) the correct direction of holding the syringe in the hand should be according to figure A (pay attention to the position of the backstop).
Priming:
· Before starting the injection, you should move the plunger slowly (with constant force) so that the gel comes out from the tip of the needle head. If the injection is done without initial slow priming, the injection pressure will increase and at the first moment, the gel will come out of the needle head with high pressure.
· Each needle can be used only for one injection and immediately after priming; if the injection is not performed immediately after priming, the gel will remain inside the needle channel, which will increase the injection pressure.
Injection:
· Injection has to be performed under aseptic conditions.
· If the needle is obstructed, do not increase the pressure on the piston, instead, replace the needle.
- Failure to take these precautionary measures can damage the needle, and/or result in a leak in the Luer-lock or an increase in the risk of vascular occlusion and inflammatory reactions.
- After inserting the needle and before the injection, it is recommended aspirate and ensure that the needle is not inserted into a blood vessel.
- In case of unusual post-injection skin whiteness, the injection should be stopped and appropriate measures, such as massaging the area until its return to natural color, should be taken.
- The quantity and duration of injection depend on the tissue requirements, tissue stress at the injection site, and the injection depth and method.
- The quantity of injection depends on the correction areas intended by the specialist.
- Excessive injection of filler for overcorrection of the shape should be avoided because an excessive injection can cause such complications as tissue necrosis and swelling.
- More treatment sessions may be needed to achieve optimal shape. Minor shape corrections (symmetry and correction of details) or repeated treatment with Fiorage may also be needed to maintain the shape.
- It is recommended to postpone the necessary correction sessions until the complications are eliminated (at least, a 2-week interval).
- Massage the treated area after the injection to better distribute the product.
- After the injection, do not apply a cold pack.
- In the event of refrigerated storage, bring the product to room temperature before injection.
Precautions for use
- Fiorage is not intended to be injected into different breast areas (breast augmentation).
- As a general principle, the infection is a prevalent complication of medical substance injection. It is thus recommended to observe standard precautions.
- Avoid injection into areas with a permanent implant.
- Clinical efficiency and tolerability of Fiorage are uncertain in patients with a history of immune deficiency or patients using immunosuppressant drugs. Specialists should make a case-by-case decision depending on the nature of the disease and associated treatment. They should also ensure accurate monitoring of patients. In particular, it is recommended that these patients undergo initial skin allergy tests and avoid injection if an allergic complication is observed. The injection should not be given to people who have a progressive autoimmune disease.
- Clinical efficiency and tolerability of Fiorage are uncertain in patients with a history of severe sensitivity (allergy) and/or anaphylactic shock. Specialists should make a case-by-case decision depending on the disease nature and associated They should also ensure accurate monitoring of patients. In particular, it is recommended that these patients undergo initial skin.
- allergy tests and/or preventative measures before injection. Fiorage injection is not recommended for patients with anaphylactic shock.
- In patients who are highly susceptible to allergies, skin diseases, hemostasis disorders or inflammatory diseases, or if the precautions for use are not followed, the incidence of side effects may increase.
- Patients with a history of streptococcal diseases (recurrent sore throat and acute rheumatic fever) should undergo skin allergy tests before any injection. The injection of Fiorage is not recommended for patients with acute rheumatic fever with heart complications.
- Patients taking anticoagulants or blood thinners (warfarin, acetylsalicylic acid, nonsteroidal anti-inflammatory drugs, any substance that prolongs blood clots, such as herbal supplements containing garlic or maidenhair, etc.) should be warned of the possibility of increased bleeding and hematoma.
- Evidence has shown that hyaluronic acid is incompatible with quaternary ammonium salts, such as benzalkonium chloride. Do not put Fiorage in contact with these substances or surgical medical instrumentation treated with such disinfectants.
- There is no information on the safety of injecting more than 20 mg cross-linked hyaluronic acid per 60 kg body weight per year.
- Given the presence of lidocaine in Fiorage, this product is not recommended for patients taking liver metabolism-reducing or inhibiting drugs.
- Due to its lidocaine content, Fiorage should be used with caution in patients with symptoms of heart disorders.
- Avoid any makeup for 12 hours after the injection.
- Avoid sun exposure, ultraviolet rays, subzero temperature, sauna, and bath within two weeks after the injection.
- The patients are recommended to use high-coverage sunscreen for two weeks after treatment.
- If the patient has a history of herpes, there is a risk that the needle puncture will trigger a new episode of herpes.
- To avoid excessive correction, inject slowly.
- Avoid injection into blood vessels.
- Avoid injection into nerves; any accidental nerve injury may cause transient paresthesia.
- It is recommended not to mix Fiorage with another product.
- Use only the needles supplied in the package in order to inject Fiorage.
- Stop the injection and remove the needle if pain increases during the injection.
- Do not use the damaged packages (syringes, medicine).
- Inject immediately after opening.
- Fiorage is designed for single use. Do not reuse this product. To avoid the risk of cross-contamination, use each syringe for only one patient.
- It is not possible to re-sterilize the product after opening even though it has not been injected.
- After injection, discard the used needles and syringes in suitable containers according to local environmental regulations.
- Fiorage contains the active ingredient lidocaine hydrochloride which can be falsely positive in some tests (such as anti-doping controls).
Dosage and instructions
- This product should only be used by experienced practitioner trained in the anatomy of the injection site and its surroundings.
- Practitioners should be aware that this product contains lidocaine.
- The product is selected by the practitioner based on the anatomy of the area and the treatment method.
Before treatment, the practitioner should:
- Thoroughly review the patient’s medical history.
- Inform the patient about the product and the expected results.
- Inform the patient about the symptoms, contraindications, incompatibilities, and side effects or dangers of injecting dermal fillers and ensure that the patient is aware of the signs and symptoms of possible side effects.
Before starting the injection:
- Thoroughly disinfect the injection area.
- Make sure that the contents of the syringe are uniform and not cloudy.
- Never try to straighten a bent needle; discard the bent needle and use a new one.
- If the product is stored in the refrigerator, maintain it at the room temperature for a while before injection.
Injection methods
- Inject slowly with the minimum pressure.
- It is recommended to use linear threading, multipoint injection, or a combination of both methods. The effectiveness is reduced if injected too deeply. Very superficial injections can result in skin discoloration or irregular correction.
- The amount of injection depends on the areas to be corrected based on the practitioner’s experience. Avoid injecting large volumes for shaping. Excessive volume injections can lead to small swellings or irregular correction.
- The amount and duration of correction of the injection site depends on the tissue requirement, the tissue stress at the injection site, and the injection depth and method.
- Avoid injecting large volumes to correct the shape. Excessive volume injections can lead to some side effects such as tissue necrosis and swelling.
- An annual volume of less than 20 mL of cross-linked hyaluronic acid is recommended for each person.
- Do not increase the pressure on the piston if the needle is blocked. Stop the injection and use another needle.
- Do not cool the treated area after injection. Massage the treated area to ensure even distribution of the substance.
Warnings
- Carefully check the expiration date.
- Do not use the cloudy and fragmented product.
- Do not use the syringe more than once. The product may no longer be sterile if reused.
- Do not re-sterilize the syringe.
- Place the used syringes in a disposal container. Repeat the instructions for other syringes.
- Do not try to fix a bent needle, instead, replace it with a new one.
- Fiorage gel should be used before the expiration date printed on the package.
- Inject immediately after opening.
- The filler should only be used on one patient to avoid any cross-contamination.
Storage condition
- Store the product at a temperature between 2°C and 25°C.
- Store away from frost and light.
- The content is fragile.
- Shelf life is 2 years, correctly preserved.
- Use the product before the expiration date on the package.


